Ionic Equilibria
Class-12-Chemistry-Chapter-3-Maharashtra Board
Notes-Part-2
Topics to be Learn : Part-2
|
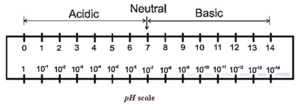
pH Scale :
Most of the chemical reactions and industrial processes are carried out in aqueous solutions, hence there is a need to know concentration of H+ and OH− ions in the solution.
Sorensen developed a convenient scale to represent the acidic, basic or neutral nature of the solution.
The pH scale is used to express the concentration of H+ and OH− along with pH and pOH of the solution.
According to Sorensen,
pH = —log10[H3O+], pOH = — log1o[OH−]
pH + pOH = 14.
Ionic product of water : It is defined as the product of molar concentrations of hydronium ions (or hydrogen ions) and hydroxyl ions at equilibrium in pure water at constant temperature.
It is represented as,
Kw = [H3O+] x [OH−]
where Kw is called ionic product of water. At 25 0C
KW = 1 x 10−14.
pH : The negative logarithm, to the base 10, of the molar concentration of hydrogen ions, H+ is known as the pH of a solution.
pH = — log10[H+]
pOH : The negative logarithm, to the base 10, of the molar concentration of hydroxyl ions, OH− is known as the pOH of a solution.
pOH = —log10[OH−]
Relationship between pH and pOH :
The ionic product of water, Kw is given by,
Kw = [H3O+] x [OH−]
At 298 K, Kw =1 x 10−14.
pKw = —log10Kw= — log1o1 x 10−14.= 14
[H3O+] x [OH−] =1 x 10−14
Taking logarithm to base 10 of both sides,
log10[H3O+] + log1o[OH−] = log1o1 x 10−14
Multiplying both the sides by — 1,
—log10[H3O+] — log1o[OH−] = —log1o1 x 10−14
‘.’ pH = —log10[H3O+], pOH = — log1o[OH−]
pKw = —log10Kw
∴ pH + pOH = pKw
Or pH + pOH = 14
Acidity, basicity and neutrality of aqueous solutions :
(i) Neutral solution : For pure water or any aqueous neutral solution at 298 K
[H3O+] = [OH− ] = 1.0 × 10-7 M
Hence, pH = -log10[H+] = -log10[1 × 10-7] = 7
(ii) Acidic solution : In acidic solution, there is excess of H3O+ ions, or [H3O+] > [OH−] Hence,
[H3O+] > 1 × 10-7 and pH < 7
(iii) Basic solution : In basic solution, the excess of OH− ions are present that is [H3O+] < [OH−]
or [H3O+] < 1.0 × 10-7 with pH > 7.
Know this :
|
Hydrolysis of salts :
Hydrolysis : A reaction in which the cations or anions or both the ions of a salt react with water to produce acidity or basicity or sometimes neutrality is called hydrolysis.
Types of salts :
These are of four types
(i) Salts derived from strong acid and strong base :
- Example : NaCl, Na2SO4, NaNO3, KCl, KNO3.
NaCl :
NaOH + HCl ⇌ NaCl + H2O
strong base strong acid salt
(ii) Salts derived from strong acids and weak bases :
- Example : NH4Cl, CuSO4, NH4NO3, CuCl2.
NH4Cl :
NH4OH + HCI ⇌ NH4Cl+ H2O
weak base strong acid salt
(iii) Salts derived from weak acids and strong bases :
- Example : CH3COONa, KCN, Na2CO3.
CH3COONa :
CH3COOH + NaOH ⇌ CH3COONa + H2O
weak acid strong base Salt
(iv) Salts derived from weak acids and weak bases :
- Example : CH3COONH4, NH4CN.
CH3COONH4 :
CH3COOH + NH4OH ⇌ CH3COONH4 + H2O
weak acid weak base salt
Remember...
|
Q. An aqueous solution of sodium chloride is neutral.
- Sodium chloride is a salt of strong acid HCl and strong base NaOH.
- In water, it reacts forming HCl and NaOH.
- As both are strong, they dissociate almost completely to liberate H+ and OH− ions, respectively.
- H+ and OH− ions combine together to form weakly dissociating H2O. As there are no free H+ ions and OH− ions, the solution is neutral and the salt does not undergo hydrolysis.
NaCl + H2O ⇌ NaOH + HCl
Ionic equation :
Na++Cl− + H2O ⇌ Na++OH− + H++Cl−
H2O ⇌ H+ + OH−
Since the solution contains equal number of H+ and OH−, it is neutral.
Hence the salt of strong acid and strong base does not undergo hydrolysis.
Hydrolysis of the salt of strong acid and weak base ;
Salt CuSO4 is obtained from strong acid H2SO4 and weak base Cu(OH)2.
When it is dissolved in water it undergoes hydrolysis as follows :
CuSO4(aq) + 2H2O(l) ⇌ Cu(OH)2 + H2SO4
salt weak base strong acid
Cu2+(aq) + SO42—(aq) +2H2O ⇌ Cu(OH)2(aq) +2H+(aq) + SO42—(aq)
Cu2+(aq) + 2H2O (l) ⇌ Cu(OH)2(aq) + 2H+(aq)
OR
Cu2+(aq) + 4H2O (l) ⇌ Cu(OH)2(aq) + 2H3O+(aq)
Since, [H3O+] > [OH—], the solution is acidic in nature.
Hydrolysis of a salt of weak acid and strong base : .
Consider a salt of weak acid and strong base like CH3COONa. In aqueous solution it undergoes hydrolysis as follows :
CH3COONa(aq) + H2O(l) ⇌ CH3COOH(aq) + NaOH(aq)
Salt weak acid strong base
CH3COO(aq) + Na+(aq) + H2O(l) ⇌ CH3COOH(aq) + Na+ + OH—(aq)
Since the base NaOH is strong, it dissociates completely while acid CH3COOH being weak dissociates partially.
Hence [OH—] > [H3O+] in the solution and the solution reacts basic.
Hydrolysis of the salt of weak acid and weak base :
Consider a salt BA of weak acid (HA) and weak base (BOH).
In aqueous solution it undergoes hydrolysis as follows :
BA(aq) + H2O(l) ⇌ BOH(aq) + HA(aq)
salt weak base weak acid
B+(aq) + A— (aq) + H2O(l) ⇌ BOH(aq) + HA(aq)
The nature of the solution will depend upon relative strength of weak acid and weak base, hence will depend upon their dissociation constants Ka and Kb.
(i) A salt of weak acid and weak base for which Ka > Kb :
Consider hydrolysis of NH4F.
NH4+(aq) + F—(aq) + H2O(l) ⇌ NH4OH + HF
weak base weak acid
Since Ka (7.2 x 10—4) for HF is greater than Kb (1.8 x 10—5) for NH4OH, the acid dissociates partially more than the base, hence,
[H3O+] > [OH—] and the solution reacts acidic after hydrolysis.
(ii) A salt of weak acid and weak base for which Ka < Kb:
Consider hydrolysis of NH4CN.
NH4+(aq) + CN—(aq) + H2O(l) ⇌ NH4OH(aq) + HCN(aq)
weak base weak acid
Since Ka (4 x 10—10) for HF is greater than Kb (1.8 x 10—5) for NH4OH, the base dissociates more than the acid, hence,
[H3O+] < [OH—] and the solution reacts basic after hydrolysis.
(ii) A salt of weak acid and weak base for which Ka = Kb :
Consider hydrolysis of CH3COONH4.
CH3COO— + NH4+ H2O ⇌ CH3COOH + NH4OH
Salt weak acid weak base
Since Ka = Kb, the weak acid CH3COOH and weak base NH4OH dissociate to the same extent, hence, [H3O+] = [OH—] and the solution reacts neutral after hydrolysis.
Buffer solutions :
Buffer solution : It is defined as a solution which resists the change in pH even after the addition of a small amount of a strong acid or a strong base or on dilution or on addition of water.
Types of buffer solutions :
(i) Acidic buffer solution ; It is a solution containing a weak acid e.g. (CH3COOH) and its salt of a strong base. e.g. (CH3COONa).
pH of an acidic buffer is given by following Henderson Hasselbalch equation,
pH = pKa + log10\(\frac{[Salt]}{[Acid]}\)
where pKa = — log10 Ka
and Ka is the dissociation constant of weak acid.
(ii) Basic buffer solutions : It is a solution containing a weak base (e. g. NH4OH) and its salt of strong acid, (e.g. NH4Cl).
pOH of a basic buffer is given by Henderson Hassebalch equation,
pOH = pKb + log10 \(\frac{[Salt]}{[Base]}\)
where pKb = — log10 Kb and
Kb is the dissociation constant of a weak base.
Mechanism of action of an acidic buffer :
(i) An acidic buffer is a mixture of a weak acid and its salt with a strong base. The weak acid dissociates feebly, but the salt dissociates almost completely.
Moreover, due to the common ions, largely supplied by the salt, dissociation of the weak acid is further suppressed.
(CH3COOH + CH3COONa) :
CH3COONa(aa) ——> CH3COO(aq) + Na+(aq) (Complete)
(ii) When a small quantity of strong acid (H+) is added to this mixture, hydrogen ions combine with acetate ions to form undissociated acetic acid. Thus, addition of an acid does not change the pH of the buffer.
H+(aq) + CH3COO(aq) ⇌ CH3 + COOH(aq)
This removal of added H+ is called reserved basicity.
(iii) When a small quantity of a strong base (OH—) is added, the hydroxide ions react with the acid producing the corresponding anions and water.
Thus, the concentrations of H+ and OH— in the solution do not change and the pH remains constant.
CH3COOH(aq) + OH—(aq) ⇌ CH3COO—(aq) + H2O(l)
This removal of added OH— is called reserved acidity.
Mechanism of action of a basic buffer :
A basic buffer solution is a solution containing a weak base and its salt with a strong acid. The weak base dissociates feebly, but the salt dissociates completely. Moreover, due to the presence of the common ion, largely supplied by the salt, the dissociation of the base is further suppressed.
(NH4OH + NH4Cl) :
NH4Cl(aq) ——> NH4+(aq) + Cl—(aq) (Complete)
When a small quantity of a strong acid is added to the solution, the hydrogen ions combine with the base producing corresponding cations and water.
Thus, the addition of an acid does not change the pH of the buffer.
H+(aq) + NH4OH(aq) ⇌ NH4+(aq) + H2O(l)
This removal of added H+ is called reserved basicity.
When a small quantity of a strong base is added, the hydroxide ions combine with NH4+ ions to form undissociated NH4OH. As a result, the hydrogen or hydroxyl ion concentration does not change. Thus, the pH of the solution does not change.
OH— (aq) + NH4+ ⇌ NH4OH(aq)
This removal of added OH— is called reserved acidity.
Properties (or advantages) of a buffer solution :
- The pH of a buffer solution is maintained appreciably constant.
- By addition of a small amount of an acid or a base pH does not change.
- On dilution with water, pH of the solution doesn’t change.
Applications of buffer solution
Buffer solution finds extensive applications in a variety of fields. Some of its applications are given.
(i) In biochemical system : pH of blood in our body is maintained at 7.36 - 7.42 due to (HCO3 + H2CO3) buffer. A mere change of 0.2 pH units can cause death. The saline solution used for intravenous injection must contain buffer system to maintain the proper pH of the blood.
(ii) Agriculture : The soils get buffered due to presence of salts such as carbonate, bicarbonate, phosphates and organic acids. Depending on pH the fertilizers are selected.
(iii) Industry : Buffers play an important role in paper, dye, ink, paint and drug industries.
(iv) Medicine : Many medicines particularly in the liquid state have a good stability and optimum activity at a definite pH, for which buffer solutions are used.
- For example penciline preparations are carried out in the presence of a buffer of sodium citrate.
- A buffer solution of magnesium citrate is prepared by adding citric acid to Mg(OH)2.
(v) Analytical chemistry : In a qualitative analysis, the precipitation of groups, the chemical tests for detection of ions, etc. are carried out at a definite pH.
- For example, precipitation of cations of IIIA are carried in the presence of a basic buffer of pH 8 — 10 is maintained with the use of (NH4OH + NH4Cl) buffer.
| Know this :
Sodium benzoate added to jams and jellies in commercial products maintains the pH constant and acts as a preservative. Hence jams and jellies are not spoiled for a very long time unlike home made products. |
Solubility product :
Solubility : It is defined as the maximum amount of a substance in moles, that can be dissolved at constant temperature to give one litre of its saturated solution.
It is expressed in moles per litre or moles per decimeter cube of a saturated solution at given temperature.
∴ Molar solubility = \(\frac{\text{Solubility in number of moles of substance}}{\text{Volume of a solution in}dm^{-3}}\)
= \(\frac{\text{Solubility in gram per}dm^{-3}}{\text{Molecular mass of substance}}\)
Relationship between solubility and solubility product :
Consider a saturated solution of a spraingly soluble; electrolyte (or salt) AxBy at a given constant temperature.
Let S mol dm-3 be the solubility of AxBy.
A following heterogeneous ionic equilibrium exists.
AxBy(s) ⇌ AxBy(dissolved) ⇌ xAy+ + yBx—
(solid) S xS yS mol dm—3
Hence final equilibrium will be,
AxBy(s) ⇌ xAy+ + yBx—
xS yS
By a law of mass action, the equilibrium constant K will be represented as,
K = \(\frac{[A^{y+}]×[B^{x-}]}{[A_xB_{y(s)}]}\)
∴ K x [AxBy(s)] = [Ay+]x x [Bx—]y
Since the active mass (concentration) of pure solid
AxBy(s) is treated as constant, [AxBy(s)] = K’
K x [AxBy(s)] = K x K’ = K(sp)
Therefore, Ksp = [Ay+]x x [Bx—]y
where Ksp is called solubility product of AxBy.
At equilibrium the concentrations are,
[Ay+] = xS mol dm-3
[Bx—] = yS mol dm-3
Ksp = [Ay+]x x [Bx—]y
= (xS)x x(yS)y
Ksp = xx.yy.(S)x+y …….(1)
Hence solubility S is given by, -
S =\((\frac{K_{sp}}{x^xy^y})^{\frac{1}{x+y}}\) mol dm-3 …..(2)
The above equations, (1) and (2) give the relationship between solubility and solubility product.
Here x and y represent number of cations and anions respectively from the electrolyte.
Q. What is the relationship between molar solubility and solubility product for salts given below :
(i) Ag2CrO4 (ii) Ca3(PO4)2 (iii) Cr(OH)3.
(i) Ag2CrO4(s) ⇌ 2Ag+(aq) + CrO42-(aq) ......( x=2, y=1)
∴ Ksp = [Ag+]2 x [CrO42-]
Ksp = xx.yy.(S)x+y
∴ Ksp = x2.y.(S)2+1 = 22 x 1 x S3 = 4S3
∴ S = \((\frac{K_{sp}}{4})^{\frac{1}{3}}\) mol dm-3
(ii) Ca3(PO4)2(s) ⇌ 3Ca2+(aq) + 2PO43-(aq) ......( x=3, y=2)
∴ Ksp = [Ca2+]3 x [PO43-]2
Ksp = xx.yy.(S)x+y = 33 x 22 x (S)3+2
= 108 S5
∴ S = \((\frac{K_{sp}}{108})^{\frac{1}{5}}\)mol dm-3
(iii) Cr(OH)3 ⇌ Cr3+ + 3OH ......( x=1, y=3)
Ksp = xx.yy.(S)x+y = 1 x 33 x (S)1+3 = 9 S4
∴ S = \((\frac{K_{sp}}{9})^{\frac{1}{4}}\) mol dm-3
Ionic product (IP) : It is defined as the product of concentrations in mol dm-3 of ions of an electrolyte in the solution and denoted by IP.
In a saturated solution,
IP = Ksp where Ksp is the solubility product of the electrolyte.
| Know this :
The process of dissolution and precipitation of sparingly soluble ionic compounds is important in our everyday life, industry and medicine. Kidney stone is developed due to the precipitation of insoluble calcium oxalate, CaC2O4. The process of tooth decay occurs due to dissolution of enamel composed of hydroxyapatite, Ca3(PO4)3OH in acidic medium. |
Common ion effect :
Common ion : An ion common to two electrolytes is called common ion.
This is generally applicable to a mixture of a strong and a weak electrolyte.
For example, a solution containing weak electrolyte CH3COOH and strong electrolyte salt CH3COONa.
CH3COONa —> CH3COO- + Na+;
CH3COOH ⇌ CH3COO- + H+
Hence CH3COOH and CH3COONa have a common ion CH3COO-
Common ion effect : The suppression of the degree of dissociation of a weak electrolyte by the addition of a strong electrolyte having an ion in common with the weak electrolyte is called common ion effect.
For example, CH3COOH and CH3COONa have common ion CH3COO-
Explanation :
A weak electrolyte dissociates partially in aqueous solution to produce cations and anions.
Equilibrium exists between ions thus formed and the undissociated molecules.
BA ⇌ B+ + A-
For such an equilibrium, the dissociation constant
K is defined as
K = \(\frac{[B^+]×[A^-]}{[BA]}\)
K is constant for the weak electrolyte at a given temperature.
Now, if another electrolyte BC or DA is added to the solution BA, having a common ion either B+ or A-, then the concentration of either B+ or A-, is increased. However, as K is always constant, the increase in the concentration of any one of the ions shifts the equilibrium to left. In other words, the dissociation of BA is suppressed. This is called common ion effect.
For example, the dissociation of a weak acid CH3COOH is suppressed by adding
CH3COONa having common ion CH3COO- .
CH3COONa —> CH3COO- + Na+;
CH3COOH ⇌ CH3COO- + H+
Common ion effect on dissociation of a weak acid :
Consider the dissociation or ionisation of a weak acid, CH3COOH in its solution.
CH3COOH(aq) ⇌ CH3COO-(aq) + H+(aq)
The dissociation constant Ka for CH3COOH will be,
Ka = \(\frac{[CH_3COO^-]×[H^+]}{[CH_3COOH]}\)
Ka is constant for CH3COOH at constant temperature.
If a strong electrolyte like salt CH3COONa is added to the solution of CH3COOH, then on dissociation it gives a common ion CH3COO-
CH3COONa —> CH3COO- + Na+;
Due to common ion CH3COO-, overall concentration of CH3COO- in the solution is increased, which increases the ratio, \(\frac{[CH_3COO^-]×[H^+]}{[CH_3COOH]}\)
In order to keep this ratio constant, the concentration of H+ is decreased, by shifting the equilibrium to the left hand side according to Le Chatelier’s principle.
Thus the ionisation of a weak acid is suppressed by a common ion.
Common ion effect on dissociation of a weak base :
Consider the dissociation or ionisation of a weak base, NH4OH in its dilute solution.
NH4OH(aq) ⇌ NH4+(aq) + OH-(aq)
The dissociation constant Kb for NH4OH will be,
Kb = \(\frac{[NH_4^+]×[OH^-]}{[NH_4OH]}\)
If a strong electrolyte like salt NH4Cl is added to the solution of NH4OH, then it gives common ion NH4+
NH4Cl ---> NH4+ + Cl-
Due to common ion NH4+ overall concentration of NH4+ is increased, which increases the ratio
[NH4+] x [OH-]/[NH4OH]
In order to keep this ratio constant, the equilibrium is shifted to the left hand side which satisfies Le Chatelier’s principle.
Thus the ionisation of a weak base is suppressed by a common ion.
Click on below link to get PDF from store :
PDF :Class-12-Chemistry-Chapter-3-Ionic Equilibria- Notes
PDF :Class-12-Chemistry-Chapter-3-Ionic Equilibria- Solution
Class -12 -Physics -All 16 Chapters Notes (16-PDF)-Rs.135
Class -12 -Physics -All 16 Chapters Solutions (16-PDF)-Rs.131
All Notes and Solutions-Class-12-Physics-16 Chapters-(32 PDF) Rs.244
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf. Previous Chapter : Chapter-2-Solutions – Online Notes Next Chapter : Chapter-4-Chemical Thermodynamics – Online Notes
